If a neutral sulfur atom gains two more valence electrons into its valence shell, then it will achieve a stable electron configuration identical to argon (Ar). The result is the sulfide anion. Sulfur is an element that can be found on Group 6 and Period 3 on the periodic table with the Atomic Mass 32 and Atomic Number 16. In an electronic configuration, the maximum number of electrons in a given shell can be obtained using the formula 2n², where n represent the number of valence shell.
The Sulfur Dioxide which is also known as Sulphur Dioxide is the entity of a bond between Sulfur and Oxygen atoms. It is known as a formula written as SO2. Here we will provide an explanation of SO2 molecular geometry, SO2 electron geometry, SO2 bond angle, and SO2 Lewis structure.
SO2 Molecular Geometry
We know that the shape which minimizes the repulsions of electronics pairs is adopted by the molecule to form the structure. The molecular shape of SO2 is same as the molecular geometry of Carbon Dioxide (CO2). We will show the bonding of SO2 without making assumption below.
O S O
Now, if we want to check the exact molecular shape of SO2, then we should understand the positions and number of electrons distributed between Sulphur and Oxygen. In the outer level, Sulphur has six electrons, and the Oxygen has four of them among which one electron is used for each bond. So total number of ten electrons in five pairs. To make bonds, four pairs are needed, so one pair remains alone. The two double bonds use two pairs each and form as a single unit.
As the single alone pair not counted in the shape’s description, we can conclude that the molecular shape of SO2 is V-Shaped or Bent. So, our first perception of the original structure does not match with the original one.
Difference of Electron Geometry Vs Molecular Geometry
Though there are so many similarities between the electron geometry and molecular geometry, there are some key differences. One of the most notable differences is that the electron geometry can be associated with one or more molecular shapes. It depends on the central atom’s structure of electrons of the molecule, while the molecular geometry depends on the other atoms too which are bonded to the central atom or the free pairs of electrons.
SO2 Electron Geometry
The electron geometry of SO2 is formed in the shape of a trigonal planner. The three pairs of bonding electrons arranged in the plane at the angle of 120-degree. As the one pair remained alone, two double pairs are bonded and form a bent shape.
SO2 Lewis structure

To create the Lewis structure of SO2, you need to arrange the eight valence electrons on the Sulphur. To design the best Lewis structure, you also need to calculate the formal charge of every atom too. You know that both the Sulphur and Oxygen has six valence electrons each. Here we have two Oxygen atoms, so a total number of valence electrons will be eighteen.
- We will place Sulphur at the center and Oxygens at outsides.
- Now we will put the pair of electrons between the atoms to create bonds.
- Now let’s calculate the formal charges.
- For Oxygen:
- No. of valence electrons = 6
- No of bonds = 2
- Lone pairs = 2
- So, Formal Charge (FC) = No. of valence electrons – No. of Bonds – 2 X (No. of lone pairs) = 6-2-(2×2) = 0
- For Sulphur:
- No. of valance electron = 6
- No. of bonds = 2
- Lone pairs = 2
- So, FC = 6-2-(2×2) = 0
- Now, we will form the structure by completing the octet with the most electronegative element O. We will place a double bond and a single lone pair with each atom of Oxygen.
- We will finish the structure by placing the remained valence electrons on the central atom. Here we have four bond pairs and four lone pairs, so total electrons used are (4+4) x 2 = 16. So, the number of remained valence electrons are 18-16 = 2. We will put these electrons on the atom of Sulphur.
- So, our final Lewis structure of SO2 will be like:
SO2 Bond Angle
Sulfur Valence Electrons For Bonding
The SO2 has a bond angle of 120-degree. One single atom of Sulphur is bonded with two atoms of Oxygen covalently. It causes a repulsion of electron pairs to form the 120-degree angle.
Is SO2 Polar or Non-Polar?
By analyzing the Lewis structure of SO2, we can see that the SO2 is asymmetrical because it contains a region with different sharing. The molecular geometry of SO2 has a bent shape which means the top has less electronegativity, and the bottom placed atoms of Oxygen have more of it. So, the conclusion is, SO2 is a Polar molecule.
Conclusion
Here, we have explained the molecular geometry, electron geometry, Lewis structure, bond angle, and polarity of SO2 (Sulfur Dioxide). You can share your thoughts for any information missed here or if you want to know more about anything. You will get a reply from the expert.
Why Carbon Is Special
There are now more than ten million organic compounds known by chemists. Many more undoubtedly exist in nature, and organic chemists are continually creating (synthesizing) new ones. Winchester rifle serial number lookup. Carbon is the only element that can form so many different compounds because each carbon atom can form four chemical bonds to other atoms, and because the carbon atom is just the right, small size to fit in comfortably as parts of very large molecules.
Sulfate Valence Electrons
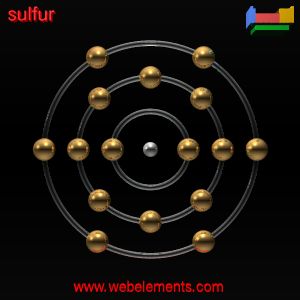
Having the atomic number 6, every carbon atom has a total of six electrons. Two are in a completed inner orbit, while the other four are valence electrons—outer electrons that are available for forming bonds with other atoms.
The carbon atom's four valence electrons can be shared by other atoms that have electrons to share, thus forming covalent (shared-electron) bonds. They can even be shared by other carbon atoms, which in turn can share electrons with other carbon atoms and so on, forming long strings of carbon atoms, bonded to each other like links in a chain. Silicon (Si), another element in group 14 of the periodic table, also has four valence electrons and can make large molecules called silicones, but its atoms are too large to fit together into as great a variety of molecules as carbon atoms can.
Carbon's ability to form long carbon-to-carbon chains is the first of five reasons that there can be so many different carbon compounds; a molecule that differs by even one atom is, of course, a molecule of a different compound. The second reason for carbon's astounding compound-forming ability is that carbon atoms can bind to each other not only in straight chains, but in complex branchings, like the branches of a tree. They can even join 'head-to-tail' to make rings of carbon atoms. There is practically no limit to the number or complexity of the branches or the number of rings that can be attached to them, and hence no limit to the number of different molecules that can be formed.
The third reason is that carbon atoms can share not only a single electron with another atom to form a single bond, but it can also share two or three electrons, forming a double or triple bond. This makes for a huge number of possible bond combinations at different places, making a huge number of different possible molecules. And a molecule that differs by even one atom or one bond position is a molecule of a different compound.
The fourth reason is that the same collection of atoms and bonds, but in a different geometrical arrangement within the molecule, makes a molecule with a different shape and hence different properties. These different molecules are called isomers. Cff explorer windows 10arrowclever.
The fifth reason is that all of the electrons that are not being used to bond carbon atoms together into chains and rings can be used to form bonds with atoms of several other elements. The most common other element is hydrogen, which makes the family of compounds known as hydrocarbons. But nitrogen, oxygen, phosphorus, sulfur, halogens, and several other kinds of atoms can also be attached as part of an organic molecule. There is a huge number of ways in which they can be attached to the carbon-atom branches, and each variation makes a molecule of a different compound. It's just as if moving a Christmas tree ornament from one branch to another created a completely different tree.
Sulfur Valence Electron Configuration
Additional topics
Sulfur Valence Electrons Octet Rule
Science EncyclopediaScience & Philosophy: Calcium Sulfate to Categorical imperativeCarbon - How Carbon Is Found, Graphite, Diamond, The Chemistry Of Carbon, Why Carbon Is Special - Classes of carbon compounds